DSC Purity Determination
DSC purity determination can be looked on as a super melting point determination that is increasingly replacing classical melting point methods. The reason for this is that it yields both more information and more accurate results. Based on our 27 years of experience of automatic purity determination, we would like to discuss the most important factors that affect this method.
Validity of the Van't Hoff equation
DSC purity determination is based on the fact that eutectic impurities lower the melting point of a eutectic system. This effect is described by the Van’t Hoff equation:
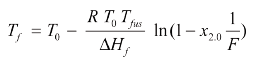
The simplified equation is:
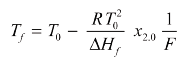
where Tf is the melting temperature (which, during melting, follows the liquidus temperature), T0 is the melting point of the pure substance, R is the gas constant, ΔHf is the molar heat of fusion (calculated from the peak area), x2.0 is the concentration (mole fraction of impurity to be determined), Tfus is the clear melting point of the impure substance, F is the fraction melted, ln is the natural logarithm, Apart is the partial area of the DSC peak, Atot is the total area of the peak and c is the linearization factor. In both cases, the reciprocal of the fraction melted (1/F) is given by the equation:
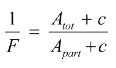
Non-eutectic impurities can also affect the melting point, and in the case of mixed crystalls, even cause an increase. Impurities that form a an empty two-component system (mutual insolubility in the liquid phase, e.g. vanilline and iron oxide) have no effect at all on the melting point of the main component...
Download the full text of this article below.
DSC Purity Determination | Thermal Analysis Application No. UC101 | Application published in METTLER TOLEDO Thermal Analysis UserCom 10