METTLER TOLEDO AutoChem offers a variety of solutions for bioprocess optimization, including in-situ probes for spectroscopy and micro-particle measurements, automated reactor workstations, and modeling software. The materials below highlight specific examples of how these solutions are being applied today by scientists at GSK, Merck, Pfizer, and others.
In-Situ Particle Measurement and Kinetics
Centrifugation and Depth Filtration Process Development

In this webinar, we explore how to conduct a rapid and data-rich study in downstream monoclonal antibody (mAb) purification.
In-Situ Spectroscopy for Concentration and Reaction Monitoring
Ultrafiltration and Diafiltration Process Development

Discover how GSK is using this innovative technology to streamline the production of mAbs and improve the overall efficiency of their downstream processing.
Real-Time Monitoring of Downstream Biologics Operations
Greg Lane of Bristol Myers Squibb details how to model critical components in TFF processes and rapidly quantify protein and excipients in real time.
Automated Reactor Workstation For Non-Sterile Biochemistry
Using EasyMax for Optimization and Scale-up of mRNA Synthesis

This free application note reviews techniques and tools for automated protocol design and execution to avoid errors caused by manual operations, ensuring consistency between synthesis batches.
Process Characterization for Low pH Virus Inactivation

This presentation discusses a full-factorial experimental design to investigate the effect of four process parameters: low pH endpoint, low pH hold time, low pH titration duration, and neutralization titration duration.
Bioprocess Modelling At Any Scale
Mechanistic Modeling of Tangential Flow Filtration to Guide Process Development
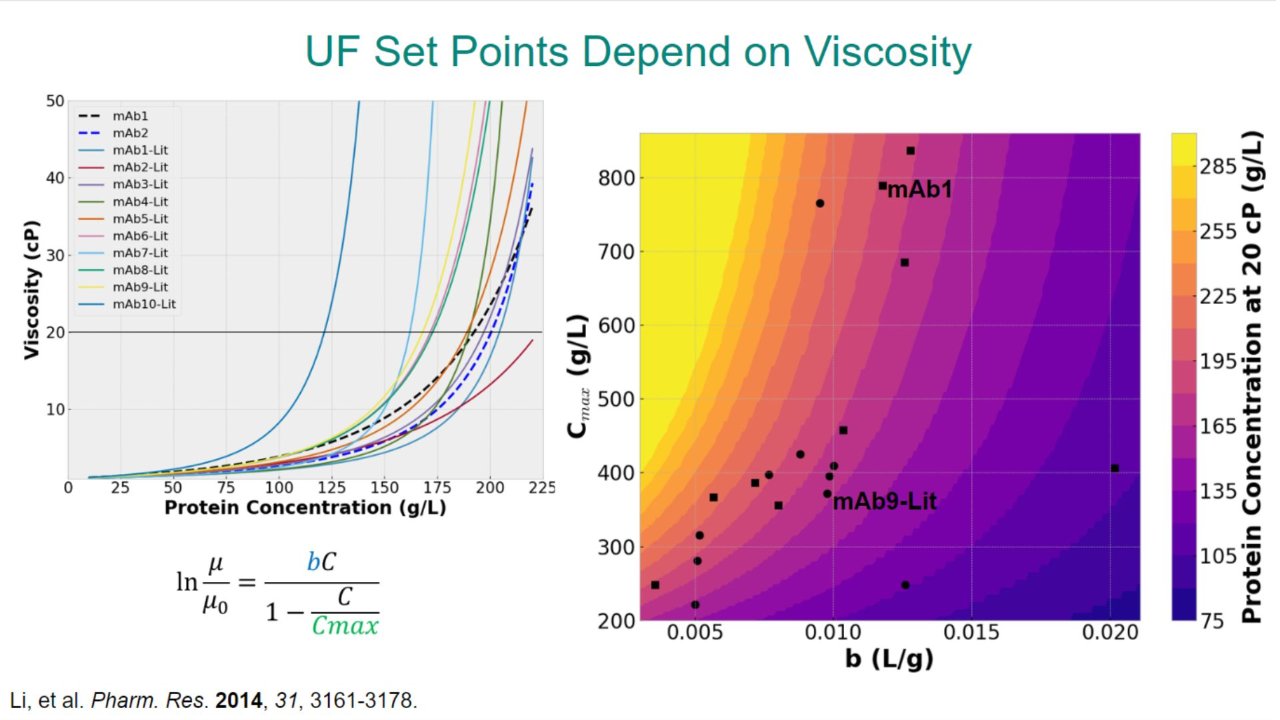
We present a modeling workflow, performed in early-stage development of UF/DF unit operations, that enables the connection of the physical components and solution properties of the DS.
Cell Culture Media Solution Preparation: In-Process Characterization and Tech Transfer to CMO

A case study regarding the incomplete dissolution of media solutions during tech transfer to CMO. Inline monitoring platform was employed to evaluate the media dissolution process at development scale.
From Experimental Data to Actionable Information
Bringing Structure to an Unstructured Lab
Hahdi Perfect | Pfizer

In this webinar, Hahdi Perfect, Senior Scientist at Pfizer, will share Pfizer’s experience of being unstructured with data to a structured approach and how it has helped improve their processes.
Demonstration of Inline Particle Analysis PAT for Bioprocess Harvesting

This online event will introduce particle system characterization PAT and automated reactor systems for experimental design and control of harvest process development.







