Yang, X., Acevedo, D., Mohammad, A., Pavurala, N., Wu, H., Brayton, A. L., Shaw, R. A., Goldman, M. J., He, F., Li, S., Fisher, R. J., O’Connor, T. F., & Cruz, C. N. (2017). Risk considerations on developing a continuous crystallization system for carbamazepine. Organic Process Research & Development, 21(7), 1021–1033. https://doi.org/10.1021/acs.oprd.7b00130
Continuous manufacturing (CM) is an emerging technology in the pharmaceutical manufacturing sector, and an understanding of the impact on product quality is evolving. As the final purification and isolation step, crystallization has a significant impact on the final physicochemical properties of drug substances and is considered a critical process step in achieving the continuous manufacturing of drug substances. Although many publications previously focused on various innovative techniques to continuously make crystals with desired properties, engineering difficulties such as system design, automation, and integration with process analytical technology (PAT) tools have not been thoroughly discussed. Here, researchers focus on how to develop a continuous crystallization system, from the perspective of process engineering and the related risk considerations on product quality.
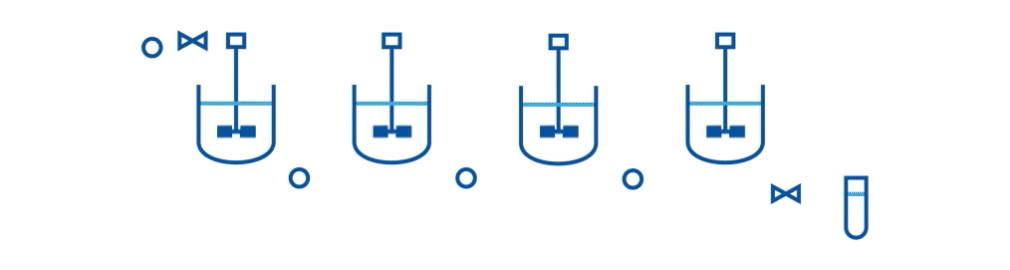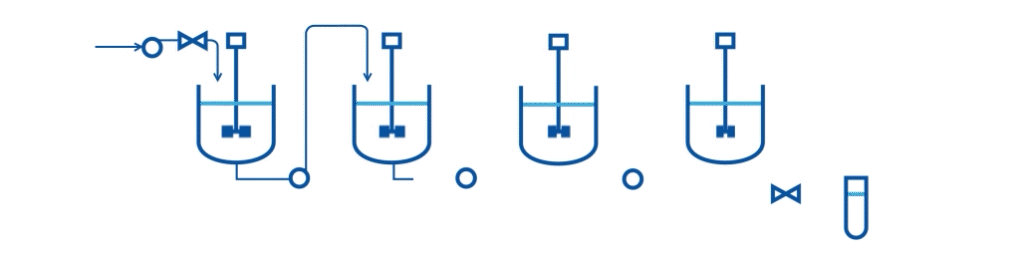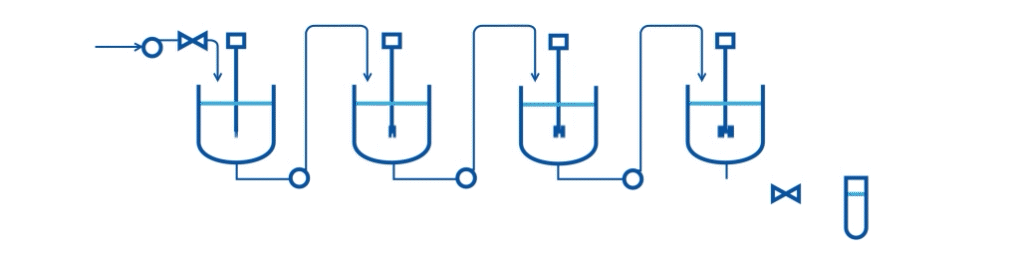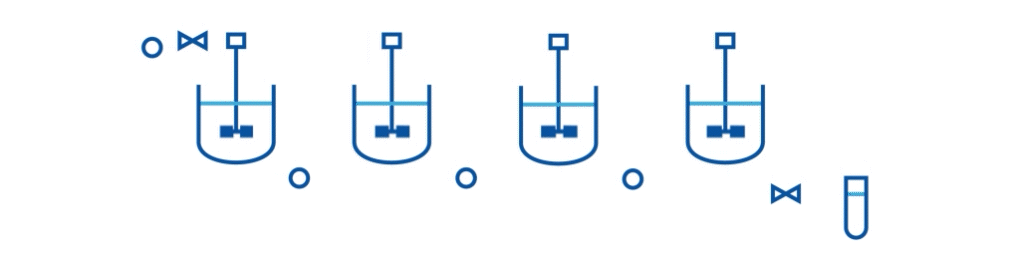
Specifically, they describe an automated two-stage mixed-suspension, mixed-product removal (MSMPR) crystallization platform for a model compound (carbamazepine, CBZ) that exhibits multiple polymorphs. The crystallization process includes the integration of PAT tools (online Raman microscopy and Focused Beam Reflectance Measurement-FBRM) for real-time monitoring.

A series of case studies were done to evaluate the performance of the continuous system and PAT tools. Specifically, the drawing schemes, slurry transport, and variations on process variables are considered the three key risk areas for continuous crystallization process development. The proof-of-concept continuous crystallization system uses feedback/feedforward controls to achieve constant levels in crystallizers, a centralized automation program, and PAT monitoring for polymorphs and particle size distribution (Raman and FBRM).
This research scale system can be used to evaluate concepts of control strategy development and process risks. Currently, the system is successfully demonstrated to perform two-stage cooling crystallization of CBZ. Preliminary studies indicate that a level control system with bottom-draw suspension removal configuration and alternating filtration setup can provide a basis of operation.