Chromatographic analysis with techniques like HPLC, UHPLC or GC is commonly used in analytical R&D and QC labs to identify, quantify and purify individual components of a mixture. Component purity, concentration and composition have a major influence on final product quality, which can impact product efficiency, safety, shelf-life, and many other important features. To obtain quantitative results, calibration standards or reference substances in known concentrations are needed.
Why weighing is important?
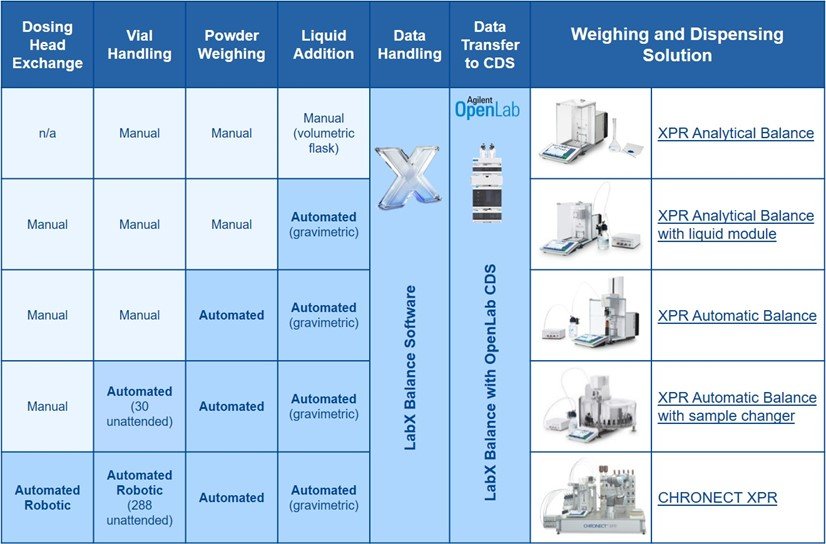
In analytical chromatography, accurate weighing, proper calculation of concentrations and error-free transfer of data have a significant impact on the quality of chromatographic results. Sample preparation can use up to 60% of analytical laboratory time and effort. Moreover, data management such as results transcription and archiving can use up to 25% of working time. Underlying the importance of state-of-the-art process automation and data management: the more tasks that can be automated and simplified without compromising accuracy, the better in terms of conserving resources and enhancing throughput and accuracy.